Background
Results from the phase 3 QuANTUM-First study (NCT02668653) showed that in nd FLT3-ITD+ AML pts, the addition of the oral, highly potent, selective, type 2 FLT3 inhibitor quizartinib (Quiz) to standard chemotherapy ± allogeneic hematopoietic cell transplantation (allo-HCT), followed by Quiz monotherapy for up to 36 cycles (3 years [yrs]), decreased the relative risk of death by 22.4% vs placebo (PBO), with a generally manageable safety profile (PMID: 37116523). We describe safety by treatment phase (Induction [IND], Consolidation [CONS], Continuation [CONT]) and by age group (<60, 60-64, ≥65 yrs).
Methods
Eligible pts (18-75 yrs) with nd FLT3-ITD+ AML were randomized 1:1 to Quiz (40 mg/d) or PBO + standard IND chemotherapy. Pts in complete remission (CR) or CR with incomplete neutrophil or platelet recovery received standard CONS chemotherapy + Quiz (40 mg/d) or PBO and/or allo-HCT (Quiz/PBO not given with allo-HCT), followed by CONT of single-agent Quiz (30-60 mg/d) or PBO for up to 36 cycles. Safety was evaluated in pts treated with ≥1 dose of Quiz or PBO. Treatment-emergent adverse events (TEAEs) were coded by MedDRA v24.0, severity by NCI-CTCAE v4.03.
Results
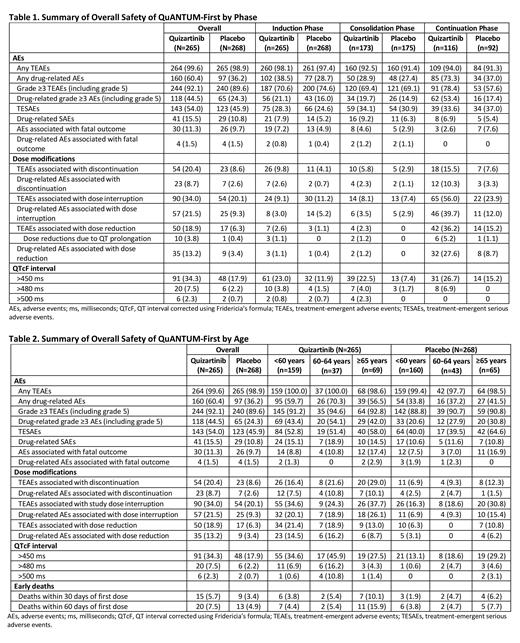
In both arms, median treatment durations were 2 weeks in IND, 4 weeks in CONS, and 1.3 yrs in CONT. Rates of TEAEs and grade ≥3 TEAEs were similar in both arms in IND & CONS, although grade ≥3 TEAEs were more common with Quiz vs PBO in CONT (Table 1). In IND & CONS, the most common TEAEs were gastrointestinal (GI) AEs (diarrhea, nausea, vomiting), infections (pneumonia, sepsis), hypokalemia, pyrexia, febrile neutropenia, and rash. In IND & CONS, myelosuppression occurred in most pts in the Quiz and PBO arms, with a median blood count recovery time <4 weeks from the time of onset. In CONT, most common TEAEs were upper respiratory tract infections, GI AEs, cytopenias, and ALT increase. In CONT, more pts with Quiz had myelosuppression, with trends toward earlier onset and delayed recovery with Quiz. ECG QT prolonged was more common with Quiz vs PBO in all phases, QTcF >500 ms was low (2.3%) and observed in IND & CONS only. Ventricular arrythmias were few with Quiz (2 pts [0.8%] had cardiac arrest/ventricular fibrillation with severe hypokalemia). Infections were the most common serious TEAEs (TESAEs), with febrile neutropenia, pneumonia, and sepsis in IND & CONS and pneumonia and viral infections in CONT. TEAEs leading to death were numerically higher with Quiz vs PBO in IND & CONS, mainly due to infections in older pts, but numerically lower with Quiz vs PBO in CONT. In IND & CONS, TEAEs leading to dose interruptions or dose reductions were similar in both arms, whereas TEAEs leading to discontinuation (disc) were higher with Quiz vs PBO. TEAEs leading to dose interruptions, dose reductions, or disc in CONT were higher with Quiz vs PBO. TEAE leading to disc of Quiz were mostly infections in IND & CONS and cytopenias in CONT. Cytopenias and QT prolonged were the most common AEs leading to dose modifications and were more common with Quiz. There was no evidence of increasing toxicity with long-term Quiz therapy for up to 36 cycles in CONT, and no new AEs were observed in pts receiving >12 cycles.
Rates of TEAEs and grade ≥3 TEAEs were similar in both arms across age groups (Table 2). TESAEs, TEAEs leading to death, and TEAEs leading to disc were more common in older pts (≥65 yrs [n=134]) vs younger pts (<60 and 60-64 yrs [n=399]) in both arms. Infections in the elderly were most commonly severe, serious, and fatal AEs and a main cause of more early deaths (<60 days of Quiz start). TEAEs leading to dose modifications were higher with Quiz vs PBO across age groups. In the Quiz arm, cytopenias, headache, rash, cough, ALT increase, hypomagnesemia, oropharyngeal pain, and back pain were more common in younger vs older pts; ECG QT prolonged was highest in the 60-64 yrs group. GI AEs and were more common in older pts with Quiz. QTcF >500 ms occurred mainly in pts 60-64 yrs with Quiz.
Conclusions
In QuANTUM-First, infections and cytopenias associated with Quiz were observed across phases. Fatal infections were more common with Quiz in IND & CONS but not in CONT. Rates of prolonged QTcF >500 ms were low overall, occurring mostly in IND & CONS, not in CONT. Pts ≥65 yrs had higher TESAEs, TEAEs leading to death (including early death), and TEAEs leading to disc (mainly infections) vs younger pts in both arms. Quiz safety profile in different treatment phases and age subgroups supports an overall positive benefit/risk.
Disclosures
Erba:Forty-Seven: Research Funding; Servier: Consultancy, Honoraria, Research Funding; Forma: Research Funding; Trillium: Consultancy; Pfizer: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Gilead: Research Funding; Sunesis Pharmaceuticals: Honoraria; Macrogenics: Consultancy, Research Funding; Syros: Consultancy; Ascentage: Research Funding; Amgen: Research Funding; ALX Oncology: Research Funding; Kura Oncology: Consultancy, Research Funding; Jazz Pharma: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Immunogen: Consultancy, Research Funding; Daiichi Sankyo Inc.: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Other: Chair, Myeloid Neoplasms Repository Study, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Agios: Consultancy, Honoraria, Research Funding; Astellas: Consultancy; Glycomimetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Other: Chair, Myeloid Neoplasms Repository Study; Genentech: Consultancy; PTE: Research Funding; Sumitomo: Research Funding. Dombret:Astellas: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees. Perl:Abbvie: Consultancy, Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Forma: Consultancy; Syndax: Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; FujiFilm: Research Funding; Foghorn: Consultancy; Beat AML: Other: Participation on a Data Safety Monitoring Board or Advisory Board; BerGen Bio: Honoraria; Genentech: Honoraria; Immunogen: Honoraria; BMS: Honoraria; Aptose: Honoraria; Rigel: Honoraria; Actinium: Honoraria. Mitov:Daiichi Sankyo Inc.: Current Employment. Liu:Daiichi Sankyo Inc.: Current Employment. Kamel:Daiichi Sankyo Inc.: Consultancy, Other. Choi:Daiichi Sankyo Inc.: Current Employment. Levis:Abbvie: Consultancy; Amgen: Consultancy; Bristol Myers Squibb: Consultancy; Daiichi-Sankyo: Consultancy; Jazz: Consultancy; Menarini: Consultancy; Takeda: Consultancy; FujiFilm: Research Funding; Pfizer: Consultancy; Astellas Global Pharma: Research Funding. Schlenk:Daiichi Sankyo: Consultancy, Honoraria, Other: Steering Committee; Receipt of equipment, materials, drugs, medical writing, gifts or other services; Pfizer: Consultancy, Honoraria, Other: Receipt of equipment, materials, drugs, medical writing, gifts or other services; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Other: Equiptment, materials medical writing, gifts, other services; PharmaMar: Other: equipment, materials medical writing, gifts, other services; AstraZeneca: Other: Receipt of equipment, materials, drugs, medical writing, gifts or other services; Boehringer Ingelheim: Other: Receipt of equipment, materials, drugs, medical writing, gifts or other services.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal